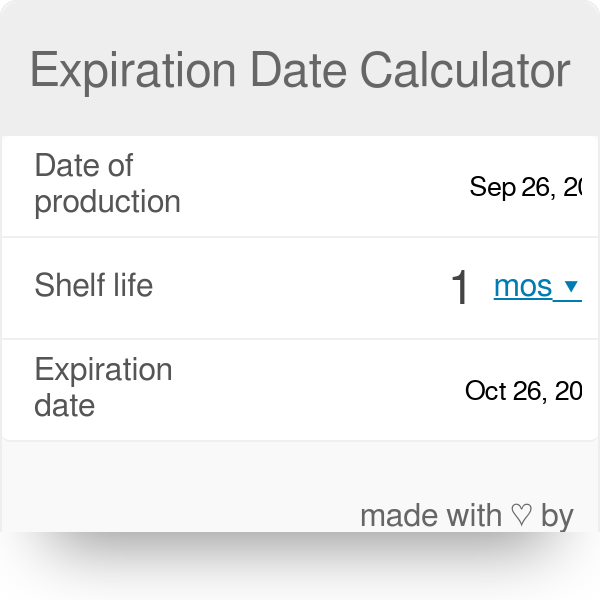
shelf life calculator for pharmaceutical products
Shelf life calculator for pharmaceutical products. The default Q10 value is set at 2.

Shelf Life Calculator For Composites And Other Materials
With the USP acceptance criteria of 100 15 from USP.

. In order to calculate expiry date you should look at Production Date on your wrapping and write it into relevant field. Batch and Product Shelf Life. After entering data you push button Check and calculator will show you if.
Input the TAA and TRT values both in Celsius for your product. Stability tests A series of tests designed to obtain information on the stability of a pharmaceutical product in order to define its shelf-life and utilization period under specified packaging and storage conditions. Understand MichaelisMenten nonlinear kinetic behavior and linearization techniques.
The batch is a single sample of the pharmaceutical products manufacturing process at. Shelf life is a product of physical microbiological and chemical processes triggered by any one of a multitude of contributing factors. A batch is a fixed quantity of product for example 100000 tablets.
These accelerated tests help pinpoint possible seal and burst strength faults leaks and film delamination in medical device and pharmaceutical packaging. New Drug Substances and Products presented at the FDA - GPhA Spring workshop in 2012 2013. θη αβNotethatαis the average drug characteristic at the time of manufacture iex 0 which is usually larger thanηThusθ0.
Stability The USP defines the stability of a pharmaceutical product as extent to which a product retains with in specified limits and throught out its period of storage and use ie its shelf life the same properties and characteristics that it possesed at the time of its manufacture. Up to 10 cash back Batch and Product Shelf Life. Click Calculate at the bottom of the calculator to determine AAT rounded up to the nearest day.
It is used to simulate real shelf-life aging and is conducted to validate shelf-life claims and document expiration dates. If youve not already done so complete the short form to see the result. Each batch is distinguished by its own shelf life which can be called the true batch shelf life.
Establishing the Shelf Life of Pharmaceutical Products. At least three distinct steps are necessary to gain knowledge and understanding of a product or process. Calculate half-life and shelf life of pharmaceutical products and drugs.
Better estimates of product shelf life 378 months disincentive for industry to include more stability batches Pharmaceutical Stability Shelf Life August 1 2010 20 3-Batch Estimate of Shelf Life n 466 18 mean 229 months SD 586 Comparison of ICH Shelf Life Estimation Methodology Using Industry Data. Supporting stability data Supplementary data such as stability data on small-scale batches related formulations. As with the single-use products data presented for bulk CRMs were acquired using bulk products stored at 4C 2C over the course of the study.
This guideline applies to human and veterinary medicines. The quality within each step influences the quality of the resulting knowledge. This online service helps you to know how long your product is in good condition.
Thus the true shelf-life denoted byθ is the solution ofηαβx hence. QUANTILE REGRESSION CALIBRATION. Depending on the response the confidence interval may be one or two sided.
Also it is necessary to find the mark. Let ˆθbe an estimator of the true shelf-lifeθbased on y. Shelf life or the expiration date of a product relative to its manufacture date when kept in the indicated environmental conditions is an essential aspect of the product lifecycle development for combination products but often lacks clarity.
What should be the accelerated stability testing and shelf-life calculation is explained in the 21 CFR part 211137- Expiration dating. To calculate the shelf life when the calculations have two limits that are both relevant to the. As a result of the publication of 21 CFR Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals requirements were outlined concerning the expiration date of a drug product and the stability testing needed to ensure that.
The shelf life for a pharmaceutical batch is defined as the maximum storage period within which the 95 confidence interval for the batch mean response level remains within the specification range. A batch is a fixed quantity of product for example 100000 tablets. Expiry with date in days in months or in years.
Understand the basis for transition-state theory and its application to chemical kinetics. A pharmaceutical product is typically manufactured in batches. Shelf life is determined by the evaluation of whole stability data of the product.
The shelf life for a pharmaceutical product is taken to be the minimum shelf life for batches on stability. Therefore developing and instituting best scientific methods at each step supports the ongoing Quality-by. Pharmaceutical industry even after sitting on the shelf for a long time or when exposed to various.
The batch is a single sample of the pharmaceutical products manufacturing process at. Long time studies on pharmaceutical products are carried out over extended time periods till the formulation fails its specifications under the recommended storage conditions. Typically storage is done at 250C - 20C and RH of 60 - 5 for up to 60 months.
A pharmaceutical product is typically manufactured in batches. The tests are prominent in biomedical research pharmaceutical and. Interpret pHrate profiles and kinetic data.
Input the Aging Factor Q10 value for your product. This document assists with establishing the expiration period of a production bath of a medicinal productIt is not applicable to biological medicinal products such as vaccines sera toxins and allergens products derived from human blood and plasma as well as medicinal products prepared biotechnologically. For details on the calculation of the shelf life for each case go to the section that describes the calculation when there is only one specification limit.
The shelf life of a drug-device combination product is determined by the shortest estimated shelf life. For this 125 mL system suitability product shelf life is defensible beyond 360 days twice the stated shelf life of 180 days. The labeled shelf life is what is printed on the drug products label and is used to calculate the expiry date.

Checking And Calculating The Shelf Life Expiration Date Sap Help Portal

Shelf Life Calculator For Composites And Other Materials

Calculation Of Expiry Date Shelf Life Of Medicine By Accelerated Stability Study Method In English Youtube
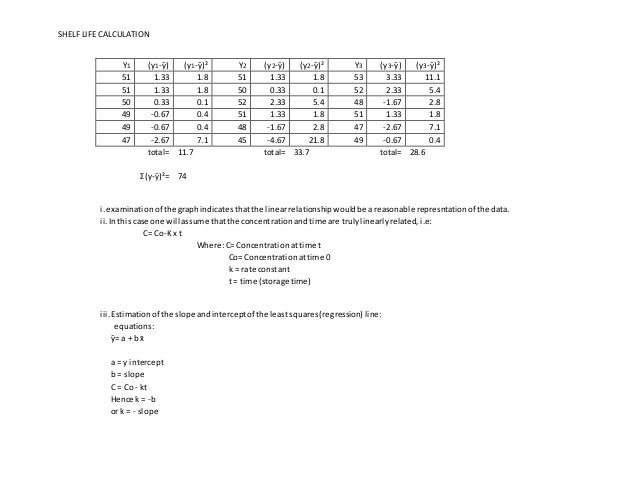
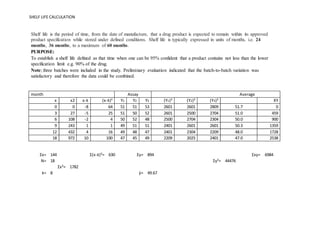
Shelf Life Calculation Of Drugs

Shelf Life Calculator For Composites And Other Materials

Food Storage Shelf Life Plus Printable Chart Food Storage Shelves Survival Food Long Term Food Storage

Evaluation Of Shelf Life Of Drug Products By Arrhenius Equation Part I Youtube

Shelf Life Calculator For Composites And Other Materials

Estimate And 95 Confidence Limits Of Shelf Life Download Scientific Diagram

Shelf Life Calculation Of Drugs

Shelf Life Of Foods First Order Kinetics Example Youtube

Shelf Life Calculation Of Drugs

Shelf Life Prediction Jmp User Community
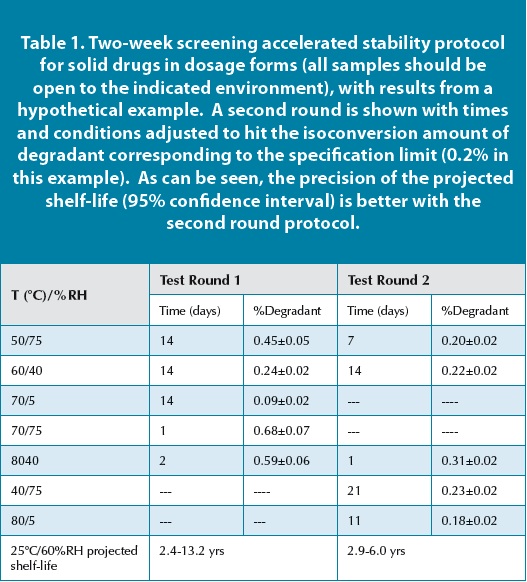
Accelerated Stability Assessment Program Asap Using Science To Set Shelf Life Pharmaceutical Outsourcing The Journal Of Pharmaceutical Biopharmaceutical Contract Services

Microsoft Excel Shelf Life Calculate On Which Day Left Equals 75 Super User

How To Calculate Expiration Dates In Excel

What To Know About The Shelf Life Of Drugs A Plus Corporation